Selected publications - Complete list available here
Click on the journal/year to access full text
Research Interests
Lab News
Research Interests
Genome integrity is maintained through faithful chromosome segregation at each cell division, in which one copy of a duplicated chromosome is deposited in each daughter cell. Errors in this process lead to aneuploidy, a condition in which cells carry an abnormal karyotype. Aneuploidy is the most common chromosome aberration in humans and is a widespread feature of solid tumors. To shed light on how aneuploidy contributes to tumorigenesis, it is crucial to determine how this condition impacts normal cells and to determine the immediate consequences of an imbalanced karyotype on cellular functions.

Spectral karyogram of a human cell
National Human Genome Research Institute

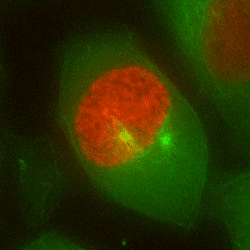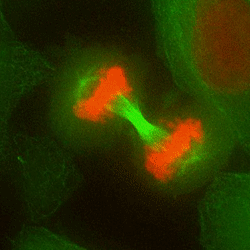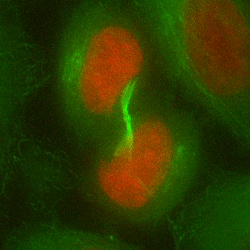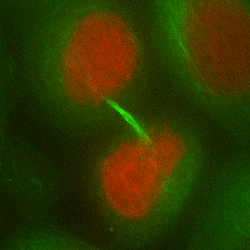

Chromosome mis-segregation in a human cell
Our work seeks to decipher how aneuploidy affects cell physiology by identifying and characterizing the pathways deregulated in human cells following chromosome segregation errors. To tackle this biological question, we use a combination of cell biology, molecular biology and genome editing techniques. Our goal is to expand our understanding of the biology of aneuploid cells and to identify specific features that can be targeted in cancer therapy.
Featured Research
Proteotoxic Stress
Proteotoxic stress is one of the most prominent feature of aneuploid cells. We are interested in deconstructing the molecular events taking place as a consequence of aneuploidy-induced proteomic changes. We also want to investigate how certain cancer cells are protected from the stresses resulting from imbalances in protein composition, with the hope to provide critical insights into how these processes can be exploited therapeutically.


Genome Instability
Aneuploidy triggers genomic instability. This ultimately leads to the evolution of cells with complex karyotypes that are cleared by immune cells in vitro. A goal of our research is to decipher the mechanisms through which aneuploidy leads to genomic instability and to determine how this continuous reshaping of the genome might influence tumor evolution. Further, our work seeks to provide the molecular details underlying immune clearance of aneuploid cells and to uncover whether and how malignant transformation can bypass immune surveillance of aneuploidy.
Synthetic Lethal Interactions with the Aneuploid State
We are interested in identifying and characterizing novel pathways critical for the survival of aneuploid cells. Toward this goal, we are developing new tools to study individual aneuploid karyotypes, in particular those that are disease-relevant. We continue to improve these systems to enable genome-wide screens, which will allow exploration of genes essential for the proliferation of aneuploid cells. This investigation will also help us defining how cancer cells are able to accommodate aneuploid karyotypes in their genomes. More broadly, we are also planning to test how individual aneuploid karyotypes are promoting proteotoxic stress, triggering immune clearance or being responsible for other phenotypes associated with the aneuploid state.

